Over the last several years, various technology experts have been discussing how the impact of big data, machine learning, and artificial intelligence will affect our daily lives – from what we buy online to how healthy we live offline. The healthcare industry has not been immune to this latest data science trend. Its goal to improve patient care while keeping spending at bay, makes it an ideal industry to benefit from the application of data science. The healthcare industry which has relied on disparate clinical trials, medical publications, patient outcomes data, case stories, or treatment patterns has for decades not been able to easily integrate and analyze this information in a unifying way. This challenge is becoming more pressing with the recent data explosion that the industry is experiencing. This type of patient-centric data, referred to as real world data (RWD), are becoming increasingly instrumental in making health care decisions. RWD informs and impacts various processes including the monitoring post-marketing drug safety and enabling decision support in clinical practice. RWD is becoming actionable real world evidence (RWE) when powered by analytics, machine learning and artificial intelligence with the potential to ultimately lead to the right treatment at the right time which translates into precision medicine. As such “Real-World Evidence (RWE) is the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD.”

Source credit: Innovation at the Intersection of Clinical Trials and Real-World Data Science to Advance Patient Care (Swift et al., 2016).
The FDA is making attempts to define RWD and RWE
When Congress passed the 21st Century Cures Act in December 2016, the bill mentioned the use of RWD to collect RWE in order to get therapies to market more efficiently and affordably for the benefit of the patient. In 2018, the FDA published a RWE framework for submitting RWE and RWD for regulatory-decision making purpose. Furthermore, the FDA released an industry guidance for how to submit documents that use Real-World Data and Real-World Evidence to the FDA related to drugs and biologics in May 2019. The draft guidance supports a range of proposals, from engaging with patient groups early in the drug development process, to re-enrolling patients from early-phase into later-phase trials. The degree of impact from the proposed changes will depend upon the clarity in the final guidance and the FDA’s willingness to enforce and/or incentivize adoption of these requirements with sponsors and research programs. As the FDA is attempting to clearly define RWD and RWE, they are stating in their Real-World Evidence Framework document the following: “Real-World Data (RWD) are data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources.” These sources can include: disease registries, medical claims and billing data, electronic health records (EHRs) data, patient-generated data and reported outcomes surveys, as well as data gathered from sources such as wearables and mobile devices.
In 2019, the FDA also released the MyStudies App code to developers which was “designed to facilitate the input of RWD directly by patients which can be linked to electronic health data supporting traditional clinical trials, pragmatic trials, and observational studies and registries.”
An uptick of RWD and RWE publications
The US government was not the only organization beginning to focus on RWE; academic institutions recognized the need to understand its implications. The number of published reports, editorials, and reviews of RWD and RWE has grown substantially in the second half of the last decade (see Figure 1). Interestingly, we observe about a tenfold increase in publications with the term RWD versus RWE, which likely is a reflection of the fact that RWD is a prerequisite of RWE. Across the various publications we see a trend in reviews about RWD or RWE, and articles related to clinical trials.
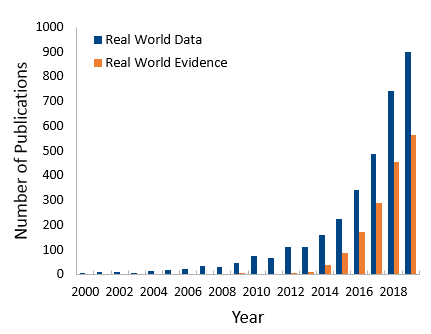
Figure 1: Real World Data and Real World Evidence mentioned in PubMed from 2000- 2019.
The Benefits of Real World Evidence
The benefits of RWD and RWE are numerous across the health care industry and we may only see the tip of the iceberg in this discussion. They touch various markets and stakeholders which includes clinicians and researchers in academia and pharma, healthcare providers, payers, and last but not least the patients. The potential for positive economics of RWD and RWE is tremendous and includes the following benefits:
- Results in personalized treatments and drug therapies for patients,
- Reveals new indications for existing therapies,
- Aids in determining more/new patient outcomes,
- Results in efficient and cost-effective clinical trials,
- Supports the integration of disparate data sources from different health systems,
- Accelerates time to market for new therapies due to the accessibility of retrospective studies, and
- May reveal new markets and previously ignored patient cohorts.
There has also been a recommendation for a new framework that transforms the mindset of the pharmaceutical industry in utilizing RWE (Olsen, 2019). The author proposes that a new framework, the evidence-powered operating framework (EPOF), would be based on seven functional pillars (from research to commercialization), and each step would utilize RWE to ultimately ensure a successful drug launch.

Source credit: Can real-world evidence save pharma us$1 billion per year? A framework for an integrated evidence generation strategy.
In addition to health systems and pharmaceutical companies, payers will also profit from the analysis of RWE. Its usage in patient care could result in more cost-effective therapies overall. RWE would allow payers to understand the effectiveness of a new drug treatment compared with the current standard of care in an eligible patient cohort. Alternatively, it would help them understand whether the treatable group is similar to the clinical trial group with respect to treatment history, socioeconomic status, or demographics. It thus almost goes without saying that all of the healthcare industries will benefit from incorporating RWE into their (business) processes.
The challenges of using RWD and RWE
With any nascent technology and capability, there are always challenges that must be addressed until the approach becomes more mature and well-understood. RWE is not only impacted by the data quality, but there are also regulatory considerations. Here is a short list of topics that may affect an organization’s incorporation of RWE into their patient care program:
- Understanding the data quality – RWD can come from EHRs, disease registries, lab reports, mobile devices data, and more – each possibly with their own proprietary format (structured versus unstructured). All of these data inputs require careful considerations when dealing with RWE.
- Data extraction and normalization – The data can be extracted automatically through natural language processing or machine learning methodologies or can be manually abstracted and categorized into a database or repository. In either case, there needs to be a standardized ontology or normalization approaches in the data processing workflow to avoid redundant and erroneous entries in the final RWD database.
- Data privacy and regulatory requirements – The rules around privacy, ethics, and usage of RWD/RWE are still being developed, and there ought to be debate even within organizations on how to manage such ambiguity.
- The usage of RWE – Organizations that use RWE need to track and monitor when RWE is being integrated (observational research or retrospective studies), how RWE is incorporated in decision making for patient care (tumor boards), and who has access to RWE information (e.g., physicians, health economists).
- Ensuring the confidence of the results – Incorporating the best analytical methodologies is essential when interpreting the results from RWD. The difficulty is deciding which novel approach or algorithms to use based on the type and quality of data being integrated.

Source credit: Privacy Analytics
Although much of the FDA and clinical attention is focused on the potential uses of RWE, more work and validation will be necessary in order to deal with the current limitations of aggregating RWD. For example, a recent paper studied the number of published clinical trials that could be feasibly replicated using observational data from electronic health records and/or insurance claims. The authors (Bartlett et. al., 2019) concluded, “Although the potential to use real-world data for RWE is substantial, the current ability to replicate the design elements from clinical trials published in the highest-impact journals may be limited. Only 15% of the 220 US-based clinical trials published in high-impact journals in 2017 could have been feasibly replicated using currently available observational data from EHRs and insurance claims.”
An active playing field for commercial RWD players
Real world data companies are keenly aware that health systems are using RWD and RWE for their precision medicine programs and that pharmaceutical and biotechnology organizations use that same RWD to drive their ongoing drug development and marketing initiatives. The investment community also believes in the strong business value of such companies and has injected hundreds of millions of dollars into new start-ups in anticipation that one of these organizations will become the industry leader in RWE delivery. Listed below, are few of the main players who provide either RWD or RWE applications and/or services to observational researchers and clinicians at health systems or pharmaceutical/biotechnology organizations. All of these companies are actively pursuing highly visible partnerships in order to gain access to large amounts of RWD or to improve their ability to analyze and interpret the disparate data sources in their own proprietary databases.
Tempus (based in Chicago) is a technology company that has clinical and molecular data to make information accessible and useful for patients, physicians, and researchers. The company has various partnerships including with CVS to launch oncology care program or ASCO to facilitate recruiting for TAPUR trial.
Syapse (based in San Francisco) delivers clinical, programmatic, and research insights from health systems, and has an ongoing research collaboration with the FDA to focus on the regulatory use of Real-World Evidence.
COTA (based in Boston) uses oncology expertise and abstraction technology to curate meaningful, longitudinal, and de-identified data to enable insight generation. Cota has an ongoing collaboration with Guardant Health to use Real-World Data to reveal insights into metastatic colon cancer and the prevalence of biomarker testing.
Flatiron (based in New York) connects community oncologists, academics, hospitals, life science researchers, and regulators via its shared technology platform. Recently, the company published study results in collaboration with Foundation Medicine on the validation of the Clinico-Genomic database as a platform to advance oncology therapeutics development and personalized cancer care.
Aetion (based in New York) developed the Aetion Evidence Platform, a scientifically validated software platform which performs rapid analyses to generate real-world evidence at scale. Aetion, IBM Watson Health Co-Developed Solution for Conducting Regulatory-Grade RWE Analysis.
IQVIA (based in Durham) uses data, technology, advanced analytics, and expertise to help customers drive healthcare. Last year, IQVIA acquired NLP provider Linguamatics.
Qiagen is a publicly held company based in Germany which not only provides sample and assay technologies for molecular diagnostics and applied testing, but it also has acquired a large number of informatics companies to enhance their portfolio of data analysis. As an example, in 2019 QIAGEN acquired N-of-One, expanding its clinical bioinformatics capabilities in molecular oncology decision support. In March 2020, it was announced that Thermo Fisher Scientific will acquired QIAGEN.
To conclude
Based on recent years’ excitement and promises around RWD and RWE, we predict that the year 2020 will see several new players, acquisitions, and alliances emerging – all attempting to address the numerous challenges of RWE. There will be more published reports that explain how best to tap into the large collection of disparate RWD sources being integrated. There will be clarity on the FDA regulation rules around incorporating RWE for clinical trials. And, if the industry is fortunate, there will be a key retrospective study that clearly illustrates how RWE played a crucial role in providing the right drug treatment for a group of patients, or how RWE was used to design successful drug trials for a pharmaceutical sponsor company. Whatever events are announced, RWE is surely not simply a buzzword in our fast-paced world of health technology. It will quickly become another invaluable tool in the healthcare industry’s quest to improve patient care and patient outcomes.
Additional RWE/RWD Publications
Real World Evidence: From market access to drug approval?
Study finds real-world evidence replicates less than one-fifth of clinical trials
Real-world evidence in clinical research: we’re not in Kansas anymore
Real World Data and Evidence Revolutionize Clinical Research

















Hi
It’s a great article.
It’s very interesting to read more about how AI can be used in the industry.
You would love to see my artificial intelligence course in delhi site as well.
Thanks once again.
[…] Real World Evidence is not simply a buzzword anymore in our fast-paced world of health technology […]